TCR challenge to FDA blackout of DCM intensifies with help from top lawyer. UPDATED
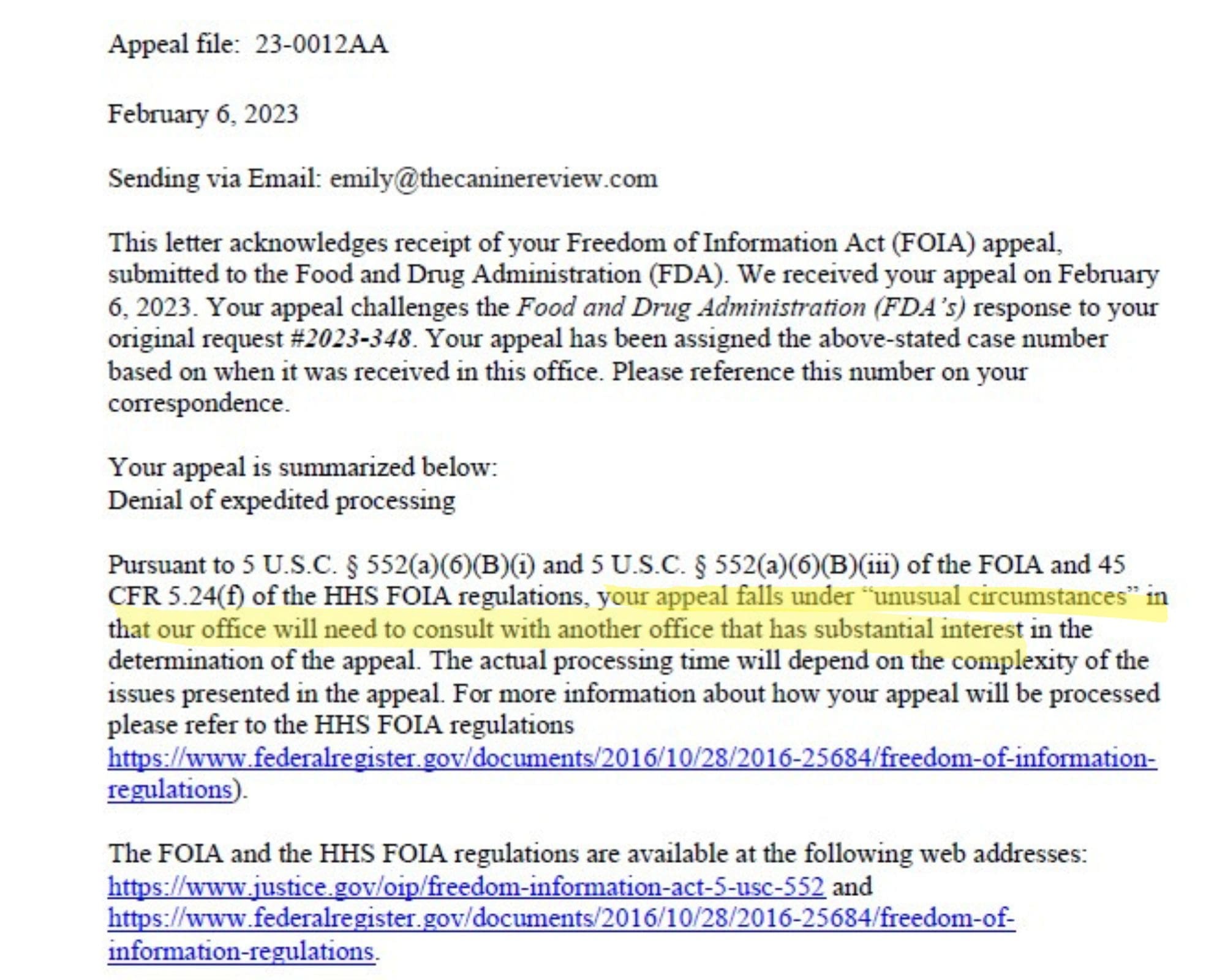
FDA says it will take 3-4 months to respond to request for expedited processing of FOIA request for DCM records!
During the same week in which the FDA told The Canine Review that the agency would need 3-4 months to respond to our appeal of its first denial (now, there are two appeals going on three) of our request for expedited processing, America’s veterinary profession’s main trade group, the AVMA, sent a grossly inaccurate story to tens of thousands of animal health professionals, declaring that the matter of diet-associated dilated cardiomyopathy in dogs was effectively closed.
The administrative appeals are a necessary and final step before suing the agency, according to constitutional law attorney David Schulz, who began assisting TCR pro bono in conjunction with Yale Law School’s Media Freedom and Information Access Clinic in November 2022.
Mr. Schulz says the conduct is typical behavior of the agency, although this case is anything but typical for him, of course: TCR is seeking records about basic animal health information for which the law clearly requires disclosure and for which the agency has never before claimed an exemption under the FOIA. The information is also frequently requested and it’s information the agency has already published in three major updates.
The FDA’s justification for denying the expedited processing is that we have not demonstrated that the matter is an urgent public concern, which we think is demonstrably absurd. Again, last week, the level of misinformation and confusion regarding canine DCM reached a new nadir when even the American Veterinary Medical Association proved unable to get its facts straight, enraging veterinary professionals.
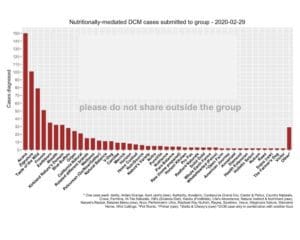
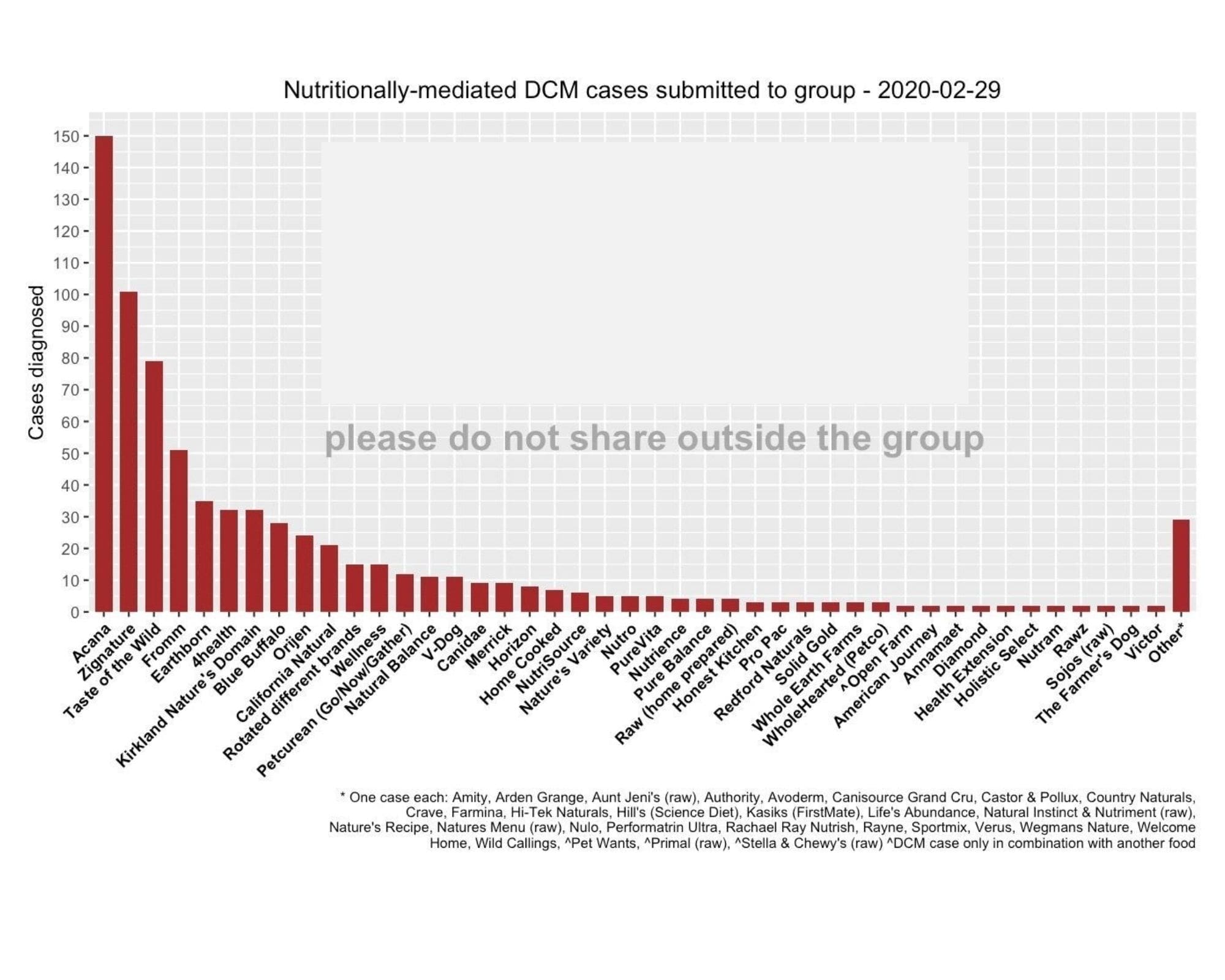
To crystallize how demonstrably absurd we believe the FDA’s justification for denying the expedited processing is — that we have not demonstrated that the matter is an urgent public concern — we are publishing the chart below. Again, last week, the level of misinformation and confusion regarding canine DCM reached a new nadir when even the American Veterinary Medical Association proved unable to get its facts straight, enraging veterinary professionals. The is an urgent matter for a significant portion of the general population (about 70 million American households include at least one dog, according to the American Pet Products Association). The data illustrates that cases of diet-associated DCM in dogs are being reported in association with some of America’s most heavily marketed, best known brands of dog food.
The Canine Review made the decision to publish this chart against the wishes of its authors for the following reasons:
- We feel strongly that the data clearly meets what the law defines as the kind of “fair use” that allows for such publication. “Fair use” is an intellectual property doctrine that justifies the use of others’ content when used for a transformative purpose — in this case, journalism on an important issue. The Canine Review is an online news service that disseminates facts and information. In the case of our reporting on diet-associated dilated cardiomyopathy in dogs, in addition to our fast-growing audience of veterinary professionals, industry leaders, and pet owners, we are also sharing the news we gather with The Washington Post.
- This chart has already been published and disseminated extensively. The chart has been available to a Facebook group with a membership of more than 126,000 individuals for more than three years.
- There is a significant and urgent public interest in the content of the chart. Many of the dog foods listed in this data are among the most popular, heavily marketed brands used by those 70 million American households, including The Farmer’s Dog, Blue Buffalo, Taste of The Wild, Acana, Merrick, Nutrisource, and many more. American consumers are largely unaware of any potential problems associated with these brands, let alone a fatal heart condition. The information void with respect to DCM that has resulted from the FDA’s unprecedented information blackout has been replaced by disinformation and, equally problematic, the sometimes unintentional parroting of that disinformation, such as the AVMA’s bungled DCM story.
Big Picture
In 2018 and 2019, the FDA’s strong warnings about “grain-free” food tried to underscore that although the exact connection had not been determined, the foods shared key commonalities. The overwhelming majority of the foods were labeled and marketed as “grain-free” and contained high concentrations of pulse ingredients like peas and lentils.
The warnings were a classic function of the FDA, much appreciated by the veterinary community who could point their clients to the FDA’s credible, authoritative assessments. The warnings were also appreciated by consumers, who would otherwise have no clue about a danger apparently lurking in what otherwise seemed to be a health-enhancing product.
However, as is now clear, these warnings were not at all appreciated by those who make their livings farming and selling peas, chickpeas, and lentil products. In the fall of 2020, the FDA’s messaging on DCM changed dramatically. Our reporting last year examined the people and interests behind that change in posture.
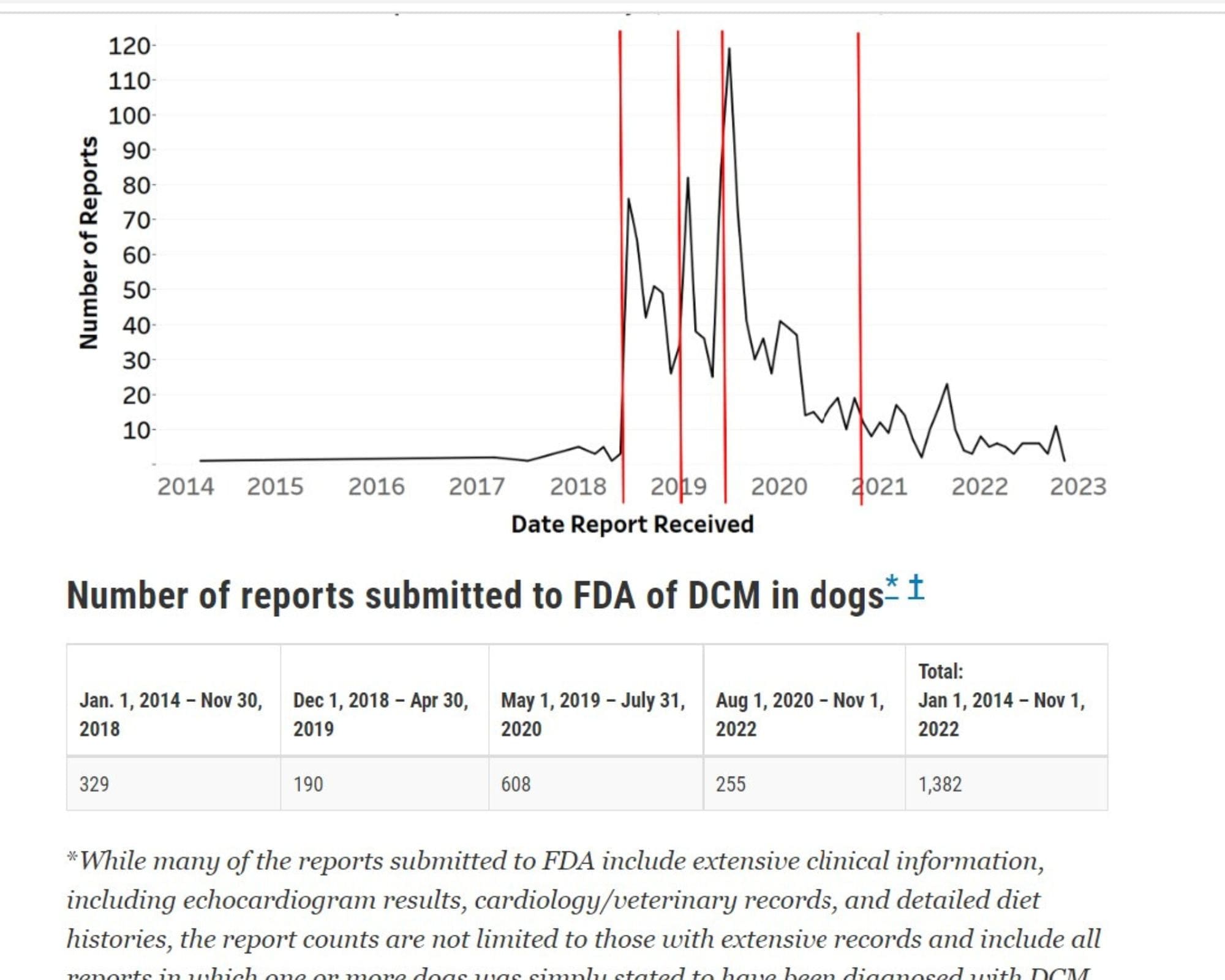
Fast forward to the end of last year, at long last, hours before Christmas Eve, after stonewalling us for nearly a full year without even a status update, the FDA updated the canine DCM case count for the first time in more than two years. Although the FDA’s stated explanation for its Dec. 2022 DCM update was “a routine update to congress,” the real reason was TCR’s longstanding Freedom of Information request, which the agency had ignored until up until legal assistance from Mr. Schulz entered the equation.
To compel the FDA to process our FOIA, Mr. Schulz advised us to withdraw formally all items we originally requested in the February 2022 FOIA apart from a single, basic piece of information: the current case count. So, that’s what the agency finally released on its website late last year. In light of the case numbers, TCR is now demanding additional agency records including the brand names reported with each case.
TCR now has a total of three new requests pending with the FDA. The request filed Monday, Feb. 6, seeks records pertaining to “remarks” made by then FDA Center for Veterinary Medicine director Dr. Steven Solomon on September 29, 2020 at a science forum on dilated cardiomyopathy (the “Remarks”). These Remarks are available online here: https://www.ksvdl.org/resources/documents/dcm-forum/DCM-Forum-SolomonOpening-Remarks.pdf. We are seeking copies of the following documents relating to the Remarks:
-
Drafts and preliminary versions of the Remarks;
-
All communications between or among Dr. Solomon and other FDA employees concerning the content of the Remarks;
-
All communications concerning the Remarks made between Dr. Solomon or any other FDA employee on the one hand and any of the following Senators or members of their staff on the other: Sen. Kevin Cramer, Sen. James Risch, Sen. Mike Crapo, Sen. Steve Daines, Sen. Roy Blunt, Sen. Jon Tester, or Sen. John Hoeven;
-
All communications concerning the Remarks made between Dr. Solomon or any other FDA employee on the one hand and any member of the U.S. House of Representatives or congressional staff on the other.
This request seeks all documents created between September 1, 2020 and December 1, 2020.
TCR has also requested a copy of the “routine update to Congress” FDA refers to in its Dec. 23, 2022 update on canine DCM: “In December 2022 as part of a routine update to Congress, FDA compiled information on canine DCM reports submitted to the agency. FDA has opted to share that information here for public awareness.”
Note: To view our FOI requests or other documents referenced, please scroll to the bottom of this article.
Chart context from group
Here is how the chart is explained by the individuals who published it who moderate this Facebook group.
Updated 2/29/20: 698 dogs diagnosed with nutritionally-mediated DCM, including 138 deaths from DCMThese are echocardiogram results reported to us by owners. Many have submitted their echocardiogram reports to veterinarians in the group for review. Many have also submitted to the FDA. All are either “confirmed” or “suspect” nutritionally-mediated DCM at this time.We have stopped showing normal echocardiogram results on this chart. The previously-charted “normal” echoes were from a few dogs that were part of the taurine table. Now that most incoming reports are DCM cases (not taurine-tested) the graphing of DCM vs normal echoes became more misleading. Additionally, our veterinarians are backlogged with the reported DCM cases and do not have time to review the “normal” echoes.This also somewhat mirrors what the FDA is doing: taking case reports, not attempting to log normal echoes. Members who have received good news are encouraged to share on the ‘Happy Hearts’ post!Key point:
– https://www.facebook.com/DietAndDCM/
Related
Which dog foods are safe? Here’s the data that fills the FDA’s DCM information void: