FDA FOIA chief promises dead-dog-heart-data-tied-to-food status update by end of week
Good news for journalists, consumers, and veterinarians. Sarah B. Kotler, the U.S. Food and Drug Administration’s Freedom of Information chief, has committed to providing all of us with a status update – it will be the first – for the Freedom of Information Act request this organization filed last February (FOIA Control # 2022-995 for anyone reading who’s worried about the moment when the data comes out – and it will – and Americans learn what was being kept from them and how many dogs were sickened or killed unecessarily).
The FOIA request was filed nearly one year ago. We’ve asked the agency to produce the number or provide a specific legal basis for delay in producing the number — that is, the number of cases FDA has received — confirmed, veterinarian-diagnosed dilated cardiomyopathy cases in dogs– since July 31, 2020
We’re not optimistic given the track record. But here’s the great news: the FOIA clinic at Yale Law School is going to help the 100 million plus Americans impacted via TCR have a judge compel the FDA to produce the information we’ve requested since the law clearly requires disclosure. In fact, the agency has not even cited a legal basis (because there isn’t one) for denying the request.
The bad news is that you’ve all had to wait this long. We can only encourage you to contact your elected, including newsly elected, representatives
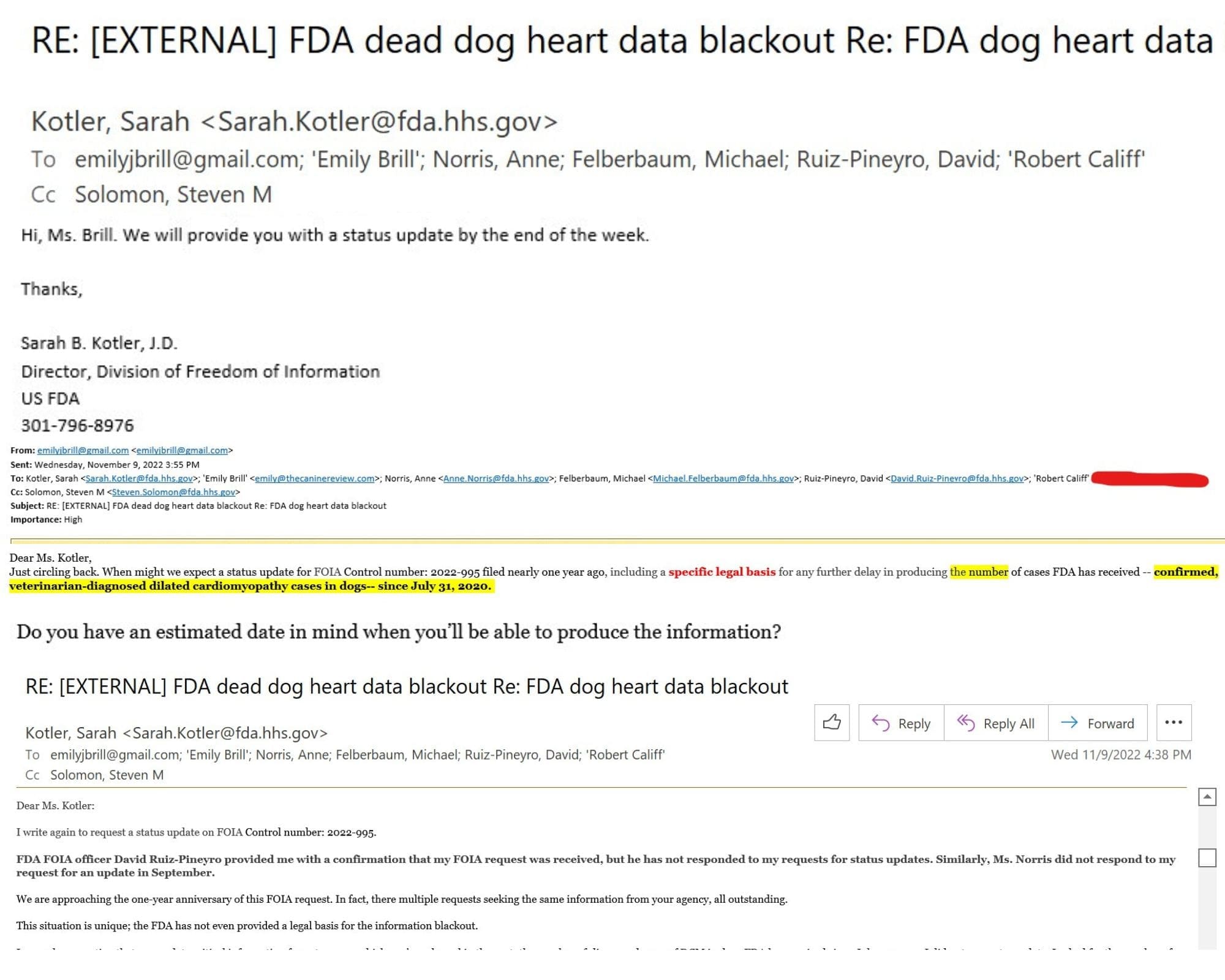
Dear Ms. Kotler:
I write again to request a status update on FOIA Control number: 2022-995.
FDA FOIA officer David Ruiz-Pineyro provided me with a confirmation that my FOIA request was received, but he has not responded to my requests for status updates. Similarly, Ms. Norris did not respond to my request for an update in September.
We are approaching the one-year anniversary of this FOIA request. In fact, there multiple requests seeking the same information from your agency, all outstanding.
This situation is unique; the FDA has not even provided a legal basis for the information blackout.
I am only requesting that you update critical information for pet owners which you’ve released in the past: the number of diagnosed cases of DCM in dogs FDA has received since July 31, 2020. I did not request raw data. I asked for the number of cases FDA has received — confirmed, veterinarian-diagnosed dilated cardiomyopathy cases in dogs– since July 31, 2020.
I note that your first warning came in 2018.
In 2019, you issued another warning, this time including a spreadsheet and naming brands most frequently appearing in reported cases.
Three years later, it seems appropriate that veterinary professionals and consumers be given access to updated data – especially in light of nearly a dozen peer reviewed studies, mounting anecdotal evidence, and because we know that a dog with nutrition DCM diagnosed early enough can be saved by changing the diet.
That prior release contained the following information:
The FDA has compiled a spreadsheet of all DCM case reports submitted through April 30, 2019. Brands named most frequently in reports submitted to the FDA (as of April 30, 2019) that had at least ten reports, include Acana (67), Zignature (64), Taste of the Wild (53), 4Health (32), Earthborn Holistic (32), Blue Buffalo (31), Nature’s Domain (29), Fromm (24), Merrick (16), California Natural (15), Natural Balance (15), Orijen (12), Nature’s Variety (11), NutriSource (10), Nutro (10), and Rachael Ray Nutrish (10). These include both grain-free and grain-containing diets in all forms (kibble, canned, raw, home-cooked). The common thread appears to be legumes, pulses (seeds of legumes), and/or potatoes as main ingredients in the food. This also includes protein, starch and fiber derivatives of these ingredients, (e.g., [source] protein, [source] starch, or [source] fiber). Some reports we have received also seem to indicate that the pets were not eating any other foods for several months to years prior to exhibiting signs of DCM.
I am simply requesting that you to update your own website, which is now providing increasingly dated information to the public.
Based on these FDA 2020 data last reported in July 2020, there is no basis not to grant the request expeditiously. This is basic vital health information that American taxpayers, including and especially America’s dog owners – more than half of U.S. households – have the right to see expeditiously.
Moreover, if you insist on continuing to withhold this essential heart data from American veterinary professionals, please cite the specific legal basis for doing so. I am bcc’ing a small group of veterinary professionals; officials at the American Veterinary Medical Association, executives of the major pet health insurance carriers (for the owners fortunate enough to get the condition diagnosed early enough, they’re not out of the woods as there is usually lifelong cardiac care required which can cost thousands of dollars or more); I’ve also looped in many of the veterinary cardiologists and nutritionists who worked with the agency on this issue.
I look forward to hearing from you.
Best regards,
Emily Brill
—
Emily Brill
Executive Editor and Founder
The Canine Review
https://www.thecaninereview.com
917-297-3537 | emily@thecaninereview.com
Date: Tuesday, September 20, 2022 at 6:16 PM
To: ‘Norris, Anne’ <Anne.Norris@fda.hhs.gov>, ‘Felberbaum, Michael’ <Michael.Felberbaum@fda.hhs.gov>, ‘Kotler, Sarah’ <sarah.kotler@fda.hhs.gov>
Cc: ‘Solomon, Steven M’ <Steven.Solomon@fda.hhs.gov>
Subject: RE: FDA dog heart data blackout
Dear Anne,
Thanks for your reply. I appreciate that you and Dr. Solomon are trying to do good work and follow the law. But the law clearly requires disclosure of this information. And, yes, I filed a FOIA request in February. #2022-995.
So, it must be the way I’m posing the question. I’ve looped in a few dozen veterinarians. Maybe one of the veterinarians copied here can pose the request even more explicitly since I’ve now been requesting this information from the Agency for nearly a full year.
I am only asking that you update critical information for pet owners which you’ve already released: the number of diagnosed cases FDA has received since July 31, 2020.
I did not ask for raw data. I asked for the number of cases FDA has received — confirmed, veterinarian-diagnosed dilated cardiomyopathy cases in dogs– since July 31, 2020.
“Diagnosed” as in, veterinarian-diagnosed.
Again: I am not asking for information which FDA has NOT already released.
I am simply asking you to update your own website, which is now providing increasingly dated information to the public, now exceeding two years.
Since you provided data just one more time in the KSU forum bringing you up to July 31, 2020, please update the website even through 2021.
Otherwise, the Agency is providing misleading information to consumers who need to read carefully enough and through the way to the 5th item to discover that the data is two years old.
Thanks,
Emily
—
Here’s the current state of the information, not updated for more than two years:
The data FDA released at a forum at KSU brings the data to July 31, 2020, which makes it more than two years old today. Note: The case numbers more than DOUBLED between 2019 at 524 and 2020 at “more than 1100,” at which point the Agency stopped releasing any data.
2020 “more than 1100 cases”
How many cases have been reported to the FDA?
Between January 1, 2014 and July 31, 2020, the FDA received more than 1100 case reports of diagnosed dilated cardiomyopathy in dogs. The majority of these cases were reported immediately after FDA provided public updates. Some of these cases involved more than one animal from the same household. In the reported cases, more than 280 of those dogs were reported to have died. Of the approximately 20 cat reports, there were approximately 13 cat deaths. The agency received additional reports of other types of cardiac disease in dogs, however, these reports did not meet our case definition requirement of a confirmed DCM diagnosis.
2019/524 cases
How many cases have been reported to the FDA? https://www.fda.gov/animal-veterinary/animal-health-literacy/questions-answers-fda-center-veterinary-medicines-investigation-possible-connection-between-diet-and
Between January 1, 2014 and April 30, 2019, the FDA received 524 case reports of diagnosed dilated cardiomyopathy. Some of these cases involved more than one animal from the same household. In the reported cases, there were 560 individual dogs diagnosed with DCM and 119 of those dogs died. There were 14 individual cats, 5 of which died. The agency received additional reports of cardiac symptoms in dogs, however, the reports did not include a confirmed DCM diagnosis.
Emily Brill
Executive Editor and Founder
The Canine Review
https://www.thecaninereview.com
917-297-3537 | emily@thecaninereview.com
From: Norris, Anne <Anne.Norris@fda.hhs.gov>
Sent: Tuesday, September 20, 2022 2:08 PM
To: emilyjbrill@gmail.com
Cc: Solomon, Steven M <Steven.Solomon@fda.hhs.gov>
Subject: RE: [EXTERNAL] RE: questions about upcoming listening session
Hi Emily,
We appreciate your interest in this issue and understand you want an updated count of DCM-related adverse events reported to the FDA. While adverse event numbers can be a potential signal of an issue with an FDA regulated product, by themselves, they do not supply relevant data to further the scientific investigation or offer actionable information to help veterinarians and pet owners protect pets. As we have previously stated, FDA isn’t planning to release an update or new numbers until there is meaningful new scientific information to share.
Thanks,
Anne